Reactions of Metals with Non-Metals
Reactions of Metals with Non-Metals: Overview
This topic covers concepts, such as, Formation of Ionic Compounds, Formation of Sodium Chloride, Electrical Conductivity of Ionic Compounds & Reaction between Metals and Non-metals etc.
Important Questions on Reactions of Metals with Non-Metals
is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state.
Compare the stability of a neutral sodium atom and a positive sodium ion. Justify your answer.
Identify the atom by its structure as shown in the first diagram, and what is being shown in the second diagram having a positive charge on it?
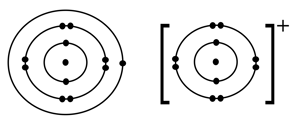
Write the cation and anion in:
The number of valence electrons in each chlorine in magnesium chloride is-
Chlorine carries a _____ charge in magnesium chloride.
Chlorine forms anions in the aqueous solution of magnesium chloride.
Explain why chlorine converts into chloride in magnesium chloride?
What is the difference between magnesium atom and magnesium ion.
The number of electrons in magnesium ion is
Magnesium ion has_____. charge.
The valency of magnesium ions is .
Write the chemical equation for the formation of magnesium ion?
Chloride ions form because of the gain of _____.
Describe the formation of magnesium chloride.
Chlorine converts to chloride ion in the formation of magnesium chloride.
The chemical equation for formation of magnesium cation is:
Ionic compounds have high melting and boiling points because there is a strong _____ force of attraction between the ions.
_____ is a metal that forms positive ions by losing two electrons(Magnesium/ Sodium).
A compound formed by the partial or complete replacement of (aq) ions of an acid by a metal ion or an electropositive ion, is called a _____.
